How To Prevent Hemolysis When Drawing Blood
BLOOD COLLECTION:
ROUTINE VENIPUNCTURE AND SPECIMEN HANDLING
Objectives for the tutorial:
-
Describe and perform the venipuncture process including:
-
Appropriate clothing and protective equipment
-
Ensuring the comfort of the patient
-
Proper patient identification procedures.
-
Proper equipment selection and use.
-
Proper labeling procedures and completion of laboratory requisitions.
-
Order of draw for multiple tube phlebotomy.
-
Preferred venous access sites, and factors to consider in site selection, and ability to differentiate between the feel of a vein, tendon and artery.
-
Patient care following completion of venipuncture.
-
Safety and infection control procedures.
-
Quality assurance issues.
-
-
Identify the additive, additive function, volume, and specimen considerations to be followed for each of the various color coded tubes.
-
List six areas to be avoided when performing venipuncture and the reasons for the restrictions.
-
Summarize the problems that may be encountered in accessing a vein, including the procedure to follow when a specimen is not obtained.
-
List several effects of exercise, posture, and tourniquet application upon laboratory values.
VENIPUNCTURE PROCEDURE
-
The venipuncture procedure is complex, requiring both knowledge and skill to perform. Each phlebotomist generally establishes a routine that is comfortable for her or him.
Phlebotomists are considered to have occupational exposure to blood borne pathogens. The performance of routine vascular access procedures by skilled phlebotomists requires, at a minimum, the use of gloves to prevent contact with blood. Laboratory coats or work smocks are not typically needed as personal protective equipment during routine venipuncture, but an employer must assess the workplace to determine whether certain tasks, workplace situations, or employee skill levels may result in an employee's need for laboratory coats or other personal protective equipment to prevent contact with blood. It is an employer's responsibility to provide, clean, repair, replace, and/or dispose of personal protective equipment/clothing. As part of presenting a professional appearance, an institutional dress code may include wearing of a laboratory coat or smock.
Several essential steps are required for every successful collection procedure:
-
Patient comfort. Is the seating comfortable and has the patient been seated for at least 5 minutes to avoid being rushed or confused?
-
Carry out hand hygiene before and after each patient procedure, before putting on and after removing gloves.
-
Identify the patient using two different identifiers, asking open ended questions such as, "What is your name?" and "What is your date of birth?"
-
Assess the patient's physical disposition (i.e. diet, exercise, stress, basal state).
-
Check the requisition form for requested tests, patient information, and any special requirements.
-
Label the collection tubes at the bedside or drawing area.
-
Select a suitable site for venipuncture.
-
Prepare the equipment, the patient and the puncture site.
-
Perform the venipuncture, collecting the sample(s) in the appropriate container(s).
-
Recognize complications associated with the phlebotomy procedure.
-
Assess the need for sample recollection and/or rejection.
-
Promptly send the specimens with the requisition to the laboratory.
-
ORDER FORM / REQUISITION
-
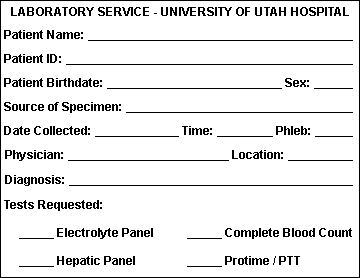
A requisition form must accompany each sample submitted to the laboratory. This requisition form must contain the proper information in order to process the specimen. The essential elements of the requisition form are:
-
Patient's surname, first name, and middle initial.
-
Patient's ID number.
-
Patient's date of birth and sex.
-
Requesting physician's complete name.
-
Source of specimen. This information must be given when requesting microbiology, cytology, fluid analysis, or other testing where analysis and reporting is site specific.
-
Date and time of collection.
-
Initials of phlebotomist.
-
Indicating the test(s) requested.
-
An example of a simple requisition form with the essential elements is shown below:
LABELING THE SAMPLE
-
A properly labeled sample is essential so that the results of the test match the patient. The key elements in labeling are:
-
Patient's surname, first and middle.
-
Patient's ID number.
-
NOTE: Both of the above MUST match the same on the requisition form.
-
Date, time and initials of the phlebotomist must be on the label of EACH tube.
-
-
Automated systems may include labels with bar codes.
-
Examples of labeled collection tubes are shown below:


EQUIPMENT:
-
THE FOLLOWING ARE NEEDED FOR ROUTINE VENIPUNCTURE:
-
Evacuated Collection Tubes - The tubes are designed to fill with a predetermined volume of blood by vacuum. The rubber stoppers are color coded according to the additive that the tube contains. Various sizes are available. Blood should NEVER be poured from one tube to another since the tubes can have different additives or coatings (see illustrations at end).
-
Needles - The gauge number indicates the bore size: the larger the gauge number, the smaller the needle bore. Needles are available for evacuated systems and for use with a syringe, single draw or butterfly system.
-
Holder/Adapter - use with the evacuated collection system.
-
Tourniquet - Wipe off with alcohol and replace frequently.
-
Alcohol Wipes - 70% isopropyl alcohol.
-
Povidone-iodine wipes/swabs - Used if blood culture is to be drawn.
-
Gauze sponges - for application on the site from which the needle is withdrawn.
-
Adhesive bandages / tape - protects the venipuncture site after collection.
-
Needle disposal unit - needles should NEVER be broken, bent, or recapped. Needles should be placed in a proper disposal unit IMMEDIATELY after their use.
-
Gloves - can be made of latex, rubber, vinyl, etc.; worn to protect the patient and the phlebotomist.
-
Syringes - may be used in place of the evacuated collection tube for special circumstances.
ORDER OF DRAW
-
Blood collection tubes must be drawn in a specific order to avoid cross-contamination of additives between tubes. The recommended order of draw for plastic collection tubes is:
-
First - blood culture bottle or tube (yellow or yellow-black top)
-
Second - coagulation tube (light blue top). If just a routine coagulation assay is the only test ordered, then a single light blue top tube may be drawn. If there is a concern regarding contamination by tissue fluids or thromboplastins, then one may draw a non-additive tube first, and then the light blue top tube.
-
Third - non-additive tube (red top)
-
Last draw - additive tubes in this order:
-
SST (red-gray or gold top). Contains a gel separator and clot activator.
-
Sodium heparin (dark green top)
-
PST (light green top). Contains lithium heparin anticoagulant and a gel separator.
-
EDTA (lavender top)
-
ACDA or ACDB (pale yellow top). Contains acid citrate dextrose.
-
Oxalate/fluoride (light gray top)
-
-
NOTE:Tubes with additives must be thoroughly mixed. Erroneous test results may be obtained when the blood is not thoroughly mixed with the additive.
PROCEDURAL ISSUES
-
PATIENT RELATIONS AND IDENTIFICATION:
-
The phlebotomist's role requires a professional, courteous, and understanding manner in all contacts with the patient. Greet the patient and identify yourself and indicate the procedure that will take place. Effective communication - both verbal and nonverbal - is essential.
-
Proper patient identification MANDATORY. If an inpatient is able to respond, ask for a full name and always check the armband or bracelet for confirmation. DO NOT DRAW BLOOD IF THE ARMBAND OR BRACELET IS MISSING. For an inpatient the nursing staff can be contacted to aid in identification prior to proceeding.
-
An outpatient must provide identification other than the verbal statement of a name. Using the requisition for reference, ask a patient to provide additional information such as a surname or birthdate. A government issued photo identification card such as a driver's license can aid in resolving identification issues.
-
If possible, speak with the patient during the process. The patient who is at ease will be less focused on the procedure. Always thank the patient and excuse yourself courteously when finished.
-
PATIENT'S BILL OF RIGHTS:
-
The Patient's Bill of Rights has been adopted by many hospitals as declared by the Joint Commission on Accreditation of Healthcare Organizations (JCAHO). The basic patient rights endorsed by the JCAHO follow in condensed form are given below.
-
The patient has the right to:
-
Impartial access to treatment or accommodations that are available or medically indicated, regardless of race, creed, sex, national origin, or sources of payment for care.
-
Considerate, respectful care.
-
Confidentiality of all communications and other records pertaining to the patient's care.
-
Expect that any discussion or consultation involving the patient's case will be conducted discretely and that individuals not directly involved in the case will not be present without patient permission.
-
Expect reasonable safety congruent with the hospital practices and environment.
-
Know the identity and professional status of individuals providing service and to know which physician or other practitioner is primarily responsible for his or her care.
-
Obtain from the practitioner complete and current information about diagnosis, treatment, and any known prognosis, in terms the patient can reasonably be expected to understand.
-
Reasonable informed participation in decisions involving the patient's health care. The patient shall be informed if the hospital proposes to engage in or perform human experimentation or other research/educational profits affecting his or her care or treatment. The patient has the right to refuse participation in such activity.
-
Consult a specialist at the patient's own request and expense.
-
Refuse treatment to the extent permitted by law.
-
Regardless of the source of payment, request and receive an itemized and detailed explanation of the total bill for services rendered in the hospital.
-
Be informed of the hospital rules and regulations regarding patient conduct.
-
-
VENIPUNCTURE SITE SELECTION:
-
Although the larger and fuller median cubital and cephalic veins of the arm are used most frequently, the basilic vein on the dorsum of the arm or dorsal hand veins are also acceptable for venipuncture. Foot veins are a last resort because of the higher probability of complications.
-
Certain areas are to be avoided when choosing a site:
-
Extensive scars from burns and surgery - it is difficult to puncture the scar tissue and obtain a specimen.
-
The upper extremity on the side of a previous mastectomy - test results may be affected because of lymphedema.
-
Hematoma - may cause erroneous test results. If another site is not available, collect the specimen distal to the hematoma.
-
Intravenous therapy (IV) / blood transfusions - fluid may dilute the specimen, so collect from the opposite arm if possible. Otherwise, satisfactory samples may be drawn below the IV by following these procedures:
-
Turn off the IV for at least 2 minutes before venipuncture.
-
Apply the tourniquet below the IV site. Select a vein other than the one with the IV.
-
Perform the venipuncture. Draw 5 ml of blood and discard before drawing the specimen tubes for testing.
-
-
Lines - Drawing from an intravenous line may avoid a difficult venipuncture, but introduces problems. The line must be flushed first. When using a syringe inserted into the line, blood must be withdrawn slowly to avoid hemolysis.
-
Cannula/fistula/heparin lock - hospitals have special policies regarding these devices. In general, blood should not be drawn from an arm with a fistula or cannula without consulting the attending physician.
-
Edematous extremities - tissue fluid accumulation alters test results.
-
-
PROCEDURE FOR VEIN SELECTION:
-
Palpate and trace the path of veins with the index finger. Arteries pulsate, are most elastic, and have a thick wall. Thrombosed veins lack resilience, feel cord-like, and roll easily.
-
If superficial veins are not readily apparent, you can force blood into the vein by massaging the arm from wrist to elbow, tap the site with index and second finger, apply a warm, damp washcloth to the site for 5 minutes, or lower the extremity over the bedside to allow the veins to fill.
-
-
PERFORMANCE OF A VENIPUNCTURE:
-
Approach the patient in a friendly, calm manner. Provide for their comfort as much as possible, and gain the patient's cooperation.
-
Identify the patient correctly.
-
Properly fill out appropriate requisition forms, indicating the test(s) ordered.
-
Verify the patient's condition. Fasting, dietary restrictions, medications, timing, and medical treatment are all of concern and should be noted on the lab requisition.
-
Check for any allergies to antiseptics, adhesives, or latex by observing for armbands and/or by asking the patient.
-
Position the patient. The patient should either sit in a chair, lie down or sit up in bed. Hyperextend the patient's arm.
-
Apply the tourniquet 3-4 inches above the selected puncture site. Do not place too tightly or leave on more than 2 minutes (and no more than a minute to avoid increasing risk for hemoconcentration). Wait 2 minutes before reapplying the tourniquet.
-
The patient should make a fist without pumping the hand.
-
Select the venipuncture site.
-
Prepare the patient's arm using an alcohol prep. Cleanse in a circular fashion, beginning at the site and working outward. Allow to air dry.
-
Grasp the patient's arm firmly using your thumb to draw the skin taut and anchor the vein. The needle should form a 15 to 30 degree angle with the surface of the arm. Swiftly insert the needle through the skin and into the lumen of the vein. Avoid trauma and excessive probing.
-
When the last tube to be drawn is filling, remove the tourniquet.
-
Remove the needle from the patient's arm using a swift backward motion.
-
Press down on the gauze once the needle is out of the arm, applying adequate pressure to avoid formation of a hematoma.
-
Dispose of contaminated materials/supplies in designated containers.
-
Mix and label all appropriate tubes at the patient bedside.
-
Deliver specimens promptly to the laboratory.
-
PHLEBOTOMY PROCEDURE ILLUSTRATED:
-
Patient identification
-
Filling out the requisition
-
Equipment
-
Apply tourniquet and palpate for vein
-
Sterilize the site
-
Insert needle
-
Drawing the specimen
-
Drawing the specimen
-
Releasing the tourniquet
-
Applying pressure over the vein
-
Applying bandage
-
Disposing needle into sharps
-
labeling the specimens
-
PERFORMANCE OF A FINGERSTICK:
-
Follow the procedure as outlined above for greeting and identifying the patient. As always, properly fill out appropriate requisition forms, indicating the test(s) ordered.
-
Verify the patient's condition. Fasting, dietary restrictions, medications, timing, and medical treatment are all of concern and should be noted on the lab requisition.
-
Position the patient. The patient should either sit in a chair, lie down or sit up in bed. Hyperextend the patient's arm.
-
The best locations for fingersticks are the 3rd (middle) and 4th (ring) fingers of the non-dominant hand. Do not use the tip of the finger or the center of the finger. Avoid the side of the finger where there is less soft tissue, where vessels and nerves are located, and where the bone is closer to the surface. The 2nd (index) finger tends to have thicker, callused skin. The fifth finger tends to have less soft tissue overlying the bone. Avoid puncturing a finger that is cold or cyanotic, swollen, scarred, or covered with a rash.
-
Using a sterile lancet, make a skin puncture just off the center of the finger pad. The puncture should be made perpendicular to the ridges of the fingerprint so that the drop of blood does not run down the ridges.
-
Wipe away the first drop of blood, which tends to contain excess tissue fluid.
-
Collect drops of blood into the collection device by gently massaging the finger. Avoid excessive pressure that may squeeze tissue fluid into the drop of blood.
-
Cap, rotate and invert the collection device to mix the blood collected.
-
Have the patient hold a small gauze pad over the puncture site for a couple of minutes to stop the bleeding.
-
Dispose of contaminated materials/supplies in designated containers.
-
Label all appropriate tubes at the patient bedside.
-
Deliver specimens promptly to the laboratory.
-
-
FINGERSTICK PROCEDURE ILLUSTRATED:
-
Equipment
-
Proper location on finger
-
Puncture with lancet
-
Drop of blood
-
Wipe first drop
-
Collecting the specimen
-
Specimen container
-
ADDITIONAL CONSIDERATIONS:
-
To prevent a hematoma:
-
Puncture only the uppermost wall of the vein
-
Remove the tourniquet before removing the needle
-
Use the major superficial veins
-
Make sure the needle fully penetrates the upper most wall of the vein. (Partial penetration may allow blood to leak into the soft tissue surrounding the vein by way of the needle bevel)
-
Apply pressure to the venipuncture site
-
-
To prevent hemolysis (which can interfere with many tests):
-
Mix tubes with anticoagulant additives gently 5-10 times
-
Avoid drawing blood from a hematoma
-
Avoid drawing the plunger back too forcefully, if using a needle and syringe, or too small a needle, and avoid frothing of the sample
-
Make sure the venipuncture site is dry
-
Avoid a probing, traumatic venipuncture
-
Avoid prolonged tourniquet application or fist clenching.
-
-
Indwelling Lines or Catheters:
-
Potential source of test error
-
Most lines are flushed with a solution of heparin to reduce the risk of thrombosis
-
Discard a sample at least three times the volume of the line before a specimen is obtained for analysis
-
-
Hemoconcentration: An increased concentration of larger molecules and formed elements in the blood may be due to several factors:
-
Prolonged tourniquet application (no more than 1 minute)
-
Massaging, squeezing, or probing a site
-
Long-term IV therapy
-
Sclerosed or occluded veins
-
-
Prolonged Tourniquet Application:
-
Primary effect is hemoconcentration of non-filterable elements (i.e. proteins). The hydrostatic pressure causes some water and filterable elements to leave the extracellular space.
-
Significant increases can be found in total protein, aspartate aminotransferase (AST), total lipids, cholesterol, iron
-
Affects packed cell volume and other cellular elements
-
Hemolysis may occur, with pseudohyperkalemia.
-
-
Patient Preparation Factors:
-
Therapeutic Drug Monitoring: different pharmacologic agents have patterns of administration, body distribution, metabolism, and elimination that affect the drug concentration as measured in the blood. Many drugs will have "peak" and "trough" levels that vary according to dosage levels and intervals. Check for timing instructions for drawing the appropriate samples.
-
Effects of Exercise: Muscular activity has both transient and longer lasting effects. The creatine kinase (CK), aspartate aminotransferase (AST), lactate dehydrogenase (LDH), and platelet count may increase.
-
Stress: May cause transient elevation in white blood cells (WBC's) and elevated adrenal hormone values (cortisol and catecholamines). Anxiety that results in hyperventilation may cause acid-base imbalances, and increased lactate.
-
Diurnal Rhythms: Diurnal rhythms are body fluid and analyte fluctuations during the day. For example, serum cortisol levels are highest in early morning but are decreased in the afternoon. Serum iron levels tend to drop during the day. You must check the timing of these variations for the desired collection point.
-
Posture: Postural changes (supine to sitting etc.) are known to vary lab results of some analytes. Certain larger molecules are not filterable into the tissue, therefore they are more concentrated in the blood. Enzymes, proteins, lipids, iron, and calcium are significantly increased with changes in position.
-
Other Factors: Age, gender, and pregnancy have an influence on laboratory testing. Normal reference ranges are often noted according to age.
-
REASONS FOR CANCELING A LABORATORY TEST
-
A test that has been ordered may be cancelled due to problems unrelated to drawing the specimen, and these are the most common causes for cancellations:
-
Duplicate test request
-
Incorrect test ordered
-
Test no longer needed
-
-
A test may be cancelled due to a technical problem in the specimen collection process:
-
Hemolysis of the specimen
-
Clotted specimen
-
Quantity of specimen not sufficient
-
Collection of specimen in incorrect tube
-
Contaminated specimen
-
Identification of the specimen is suspect
-
Delay in transport - specimen too old
-
SAFETY AND INFECTION CONTROL
-
Because of contacts with sick patients and their specimens, it is important to follow safety and infection control procedures.
-
PROTECT YOURSELF
-
Practice universal precautions:
-
Wear gloves and a lab coat or gown when handling blood/body fluids.
-
Change gloves after each patient or when contaminated.
-
Wash hands frequently.
-
Dispose of items in appropriate containers.
-
-
Dispose of needles immediately upon removal from the patient's vein. Do not bend, break, recap, or resheath needles to avoid accidental needle puncture or splashing of contents.
-
Clean up any blood spills with a disinfectant such as freshly made 10% bleach.
-
If you stick yourself with a contaminated needle:
-
Remove your gloves and dispose of them properly.
-
Squeeze puncture site to promote bleeding.
-
Wash the area well with soap and water.
-
Record the patient's name and ID number.
-
Follow institution's guidelines regarding treatment and follow-up.
-
NOTE: The use of prophylactic zidovudine following blood exposure to HIV has shown effectiveness (about 79%) in preventing seroconversion
-
-
-
PROTECT THE PATIENT
-
Place blood collection equipment away from patients, especially children and psychiatric patients.
-
Practice hygiene for the patient's protection. When wearing gloves, change them between each patient and wash your hands frequently. Always wear a clean lab coat or gown.
-
TROUBLESHOOTING GUIDELINES:
-
IF AN INCOMPLETE COLLECTION OR NO BLOOD IS OBTAINED:
-
Change the position of the needle. Move it forward (it may not be in the lumen)

-
or move it backward (it may have penetrated too far).

-
Adjust the angle (the bevel may be against the vein wall).

-
Loosen the tourniquet. It may be obstructing blood flow.
-
Try another tube. Use a smaller tube with less vacuum. There may be no vacuum in the tube being used.
-
Re-anchor the vein. Veins sometimes roll away from the point of the needle and puncture site.
-
Have the patient make a fist and flex the arm, which helps engorge muscles to fill veins.
-
Pre-warm the region of the vein to reduce vasoconstriction and increase blood flow.
-
Have the patient drink fluids if dehydrated.
-
-
IF BLOOD STOPS FLOWING INTO THE TUBE:
-
The vein may have collapsed; resecure the tourniquet to increase venous filling. If this is not successful, remove the needle, take care of the puncture site, and redraw.

-
The needle may have pulled out of the vein when switching tubes. Hold equipment firmly and place fingers against patient's arm, using the flange for leverage when withdrawing and inserting tubes.
-
-
PROBLEMS OTHER THAN AN INCOMPLETE COLLECTION:
-
A hematoma forms under the skin adjacent to the puncture site - release the tourniquet immediately and withdraw the needle. Apply firm pressure.
Hematoma formation is a problem in older patients.

-
The blood is bright red (arterial) rather than venous. Apply firm pressure for more than 5 minutes.

-
BLOOD COLLECTION ON BABIES:
-
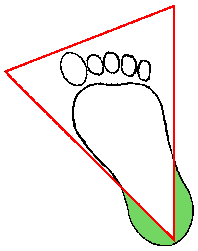
The recommended location for blood collection on a newborn baby or infant is the heel. The diagram below indicates in green the proper area to use for heel punctures for blood collection:
-
Prewarming the infant's heel (42 C for 3 to 5 minutes) is important to obtain capillary blood gas samples and warming also greatly increases the flow of blood for collection of other specimens. However, do not use too high a temperature warmer, because baby's skin is thin and susceptible to thermal injury.
-
Clean the site to be punctured with an alcohol sponge. Dry the cleaned area with a dry cotton sponge. Hold the baby's foot firmly to avoid sudden movement.
-
Using a sterile blood lancet, puncture the side of the heel in the appropriate regions shown above in green. Do not use the central portion of the heel because you might injure the underlying bone, which is close to the skin surface. Do not use a previous puncture site. Make the cut across the heelprint lines so that a drop of blood can well up and not run down along the lines.
-
Wipe away the first drop of blood with a piece of clean, dry cotton. Since newborns do not often bleed immediately, use gentle pressure to produce a rounded drop of blood. Do not use excessive pressure or heavy massaging because the blood may become diluted with tissue fluid.
-
Fill the capillary tube(s) or micro collection device(s) as needed.
-
When finished, elevate the heel, place a piece of clean, dry cotton on the puncture site, and hold it in place until the bleeding has stopped.
-
Be sure to dispose of the lancet in the appropriate sharps container. Dispose of contaminated materials in appropriate waste receptacles. Remove your gloves and wash your hands.
-
HEELSTICK PROCEDURE ILLUSTRATED:
-
Heelstick on baby
-
PEDIATRIC PHLEBOTOMY:
-
Children, particularly under the age of 10, may experience pain and anxiety during the phlebotomy procedure.
-
A variety of techniques can be employed to reduce pain and anxiety. Effective methods use distraction. These may include listening to music or a story, watching a video, playing with a toy, having a parent provide distraction with talk or touch, using flash cards, and squeezing a rubber ball. (Uman et al, 2013)
COLLECTION TUBES FOR PHLEBOTOMY
-
Collection tubes can vary in size for volume of blood drawn, appropriate to the tests ordered with sample size required, and vary in the kind of additive for anticoagulation, separation of plasma, or preservation of analyte. Larger tube sizes typically provide for collection of samples from 6 to 10 mL.

-
Smaller collection tubes for sample sizes of 2 mL or less may be appropriate in situations where a smaller amount blood should be drawn, as in pediatric patients, or to minimize hemolysis during collection, or to avoid insufficient sample volume in the collection tube.

| Red Top |  |
|---|---|
| ADDITIVE | None |
| MODE OF ACTION | Blood clots, and the serum is separated by centrifugation |
| USES | Chemistries, Immunology and Serology, Blood Bank (Crossmatch) |
| Gold Top |  |
|---|---|
| ADDITIVE | None |
| MODE OF ACTION | Serum separator tube (SST) contains a gel at the bottom to separate blood from serum on centrifugation |
| USES | Chemistries, Immunology and Serology |
| Light Green Top |  |
|---|---|
| ADDITIVE | Plasma Separating Tube (PST) with Lithium heparin |
| MODE OF ACTION | Anticoagulates with lithium heparin; Plasma is separated with PST gel at the bottom of the tube |
| USES | Chemistries |
| Purple Top |  |
|---|---|
| ADDITIVE | EDTA |
| MODE OF ACTION | Forms calcium salts to remove calcium |
| USES | Hematology (CBC) and Blood Bank (Crossmatch); requires full draw - invert 8 times to prevent clotting and platelet clumping |
| Light Blue Top |  |
|---|---|
| ADDITIVE | Sodium citrate |
| MODE OF ACTION | Forms calcium salts to remove calcium |
| USES | Coagulation tests (protime and prothrombin time), full draw required |
| Green Top |  |
|---|---|
| ADDITIVE | Sodium heparin or lithium heparin |
| MODE OF ACTION | Inactivates thrombin and thromboplastin |
| USES | For lithium level, use sodium heparin For ammonia level, use sodium or lithium heparin |
| Dark Blue Top |  |
|---|---|
| ADDITIVE | EDTA- |
| MODE OF ACTION | Tube is designed to contain no contaminating metals |
| USES | Trace element testing (zinc, copper, lead, mercury) and toxicology |
| Light Gray Top |  |
|---|---|
| ADDITIVE | Sodium fluoride and potassium oxalate |
| MODE OF ACTION | Antiglycolytic agent preserves glucose up to 5 days |
| USES | Glucoses, requires full draw (may cause hemolysis if short draw) |
| Yellow Top |  |
|---|---|
| ADDITIVE | ACD (acid-citrate-dextrose) |
| MODE OF ACTION | Complement inactivation |
| USES | HLA tissue typing, paternity testing, DNA studies |
| Yellow - Black Top |  |
|---|---|
| ADDITIVE | Broth mixture |
| MODE OF ACTION | Preserves viability of microorganisms |
| USES | Microbiology - aerobes, anaerobes, fungi |
| Black Top |  |
|---|---|
| ADDITIVE | Sodium citrate (buffered) |
| MODE OF ACTION | Forms calcium salts to remove calcium |
| USES | Westergren Sedimentation Rate; requires full draw |
| Orange Top |  |
|---|---|
| ADDITIVE | Thrombin |
| MODE OF ACTION | Quickly clots blood |
| USES | STAT serum chemistries |
| Light Brown Top |  |
|---|---|
| ADDITIVE | Sodium heparin |
| MODE OF ACTION | Inactivates thrombin and thromboplastin; contains virtually no lead |
| USES | Serum lead determination |
| Pink Top |  |
|---|---|
| ADDITIVE | Potassium EDTA |
| MODE OF ACTION | Forms calcium salts |
| USES | Immunohematology |
| White Top |  |
|---|---|
| ADDITIVE | Potassium EDTA |
| MODE OF ACTION | Forms calcium salts |
| USES | Molecular/PCR and bDNA testing |
References
-
Giavarina D, Lippi G. Blood venous sample collection: Recommendations overview and a checklist to improve quality. Clin Biochem. 2017;50(10-11):568-573.
Kiechle FL. So You're Going to Collect a Blood Specimen: An Introduction to Phlebotomy, 13th Edition (2010), College of American Pathologists, Northfield, IL.
Dalal BI, Brigden ML. Factitious biochemical measurements resulting from hematologic conditions. Am J Clin Pathol. 2009 Feb;131(2):195-204.
Lippi G, Salvagno GL, Montagnana M, Franchini M, Guidi GC. Phlebotomy issues and quality improvement in results of laboratory testing. Clin Lab. 2006;52(5-6):217-30.
Lippi G, Blanckaert N, Bonini P, Green S, Kitchen S, Palicka V, Vassault AJ, Mattiuzzi C, Plebani M. Causes, consequences, detection, and prevention of identification errors in laboratory diagnostics. Clin Chem Lab Med. 2009;47(2):143-53.
Occupational Safety and Health Administration, United States Department of Labor. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=INTERPRETATIONS&p_id=25913 and https://www.osha.gov/OshDoc/data_BloodborneFacts/bbfact03.pdf (Accessed January 10, 2018).
Phelan MP, Reineks EZ, Berriochoa JP, Schold JD, Hustey FM, Chamberlin J, Kovach A. Impact of Use of Smaller Volume, Smaller Vacuum Blood Collection Tubes on Hemolysis in Emergency Department Blood Samples. Am J Clin Pathol. 2017;148(4):330-335.
Uman LS, Birnie KA, Noel M, et al. Psychological interventions for needle-related procedural pain and distress in children and adolescents. Cochrane Database Syst Rev. 2013 Oct 10;(10):CD005179. doi: 10.1002/14651858.CD005179.pub3.
Valenstein PN, Sirota RL. Identification errors in pathology and laboratory medicine. Clin Lab Med. 2004;24(4):979-96, vii.
World Health Organization. WHO guidelines on drawing blood: best practices in phlebotomy. https://www.ncbi.nlm.nih.gov/books/NBK138650/pdf/Bookshelf_NBK138650.pdf (Accessed January 10, 2018)
And for our furry friends:
Joslin JO. Blood Collection Techniques in Exotic Small Mammals. Journal of Exotic Pet Medicine. 2009;18(2):117-139.
How To Prevent Hemolysis When Drawing Blood
Source: https://webpath.med.utah.edu/TUTORIAL/PHLEB/PHLEB.html
Posted by: lynnfouty1959.blogspot.com

0 Response to "How To Prevent Hemolysis When Drawing Blood"
Post a Comment