How To Draw Radial Wave Function
8.two: The Wavefunctions
- Page ID
- 4528
The solutions to the hydrogen atom Schrödinger equation are functions that are products of a spherical harmonic office and a radial function.
\[ \psi _{n, l, m_l } (r, \theta , \varphi) = R_{n,fifty} (r) Y^{m_l}_l (\theta , \varphi) \label {8-20}\]
The wavefunctions for the hydrogen atom depend upon the three variables r, \(\theta\), and \(\varphi \) and the three quantum numbers due north, \(fifty\), and \(m_l\). The variables give the position of the electron relative to the proton in spherical coordinates. The absolute square of the wavefunction, \(| \psi (r, \theta , \varphi )|^2\), evaluated at \(r\), \(\theta \), and \(\varphi\) gives the probability density of finding the electron within a differential volume \(d \tau\), centered at the position specified by r, \(\theta \), and \(\varphi\).
Do \(\PageIndex{1}\)
What is the value of the integral
\[ \int \limits _{\text{all space}} | \psi (r, \theta , \varphi )|^2 d \tau \, ? \nonumber\]
The quantum numbers accept names: \(n\) is called the main quantum number, \(l\) is chosen the athwart momentum quantum number, and \(m_l\) is called the magnetic breakthrough number because (as we will run across in Section 8.4) the energy in a magnetic field depends upon \(m_l\). Often \(fifty\) is chosen the azimuthal breakthrough number because it is a consequence of the \(\theta\)-equation, which involves the azimuthal angle \(\Theta \), referring to the angle to the zenith.
These quantum numbers have specific values that are dictated by the physical constraints or boundary weather condition imposed upon the Schrödinger equation: \(n\) must be an integer greater than 0, \(l\) can have the values 0 to n‑1, and \(m_l\) can have \(2l + 1\) values ranging from \(-l\) ‑ to \(+l\) in unit or integer steps. The values of the quantum number \(l\) usually are coded by a letter: s ways 0, p means i, d means 2, f means three; the next codes continue alphabetically (e.one thousand., one thousand means \(50 = 4\)). The quantum numbers specify the quantization of physical quantities. The detached energies of different states of the hydrogen atom are given past \(n\), the magnitude of the angular momentum is given by \(l\), and one component of the athwart momentum (usually chosen past chemists to exist the z‑component) is given by \(m_l\). The total number of orbitals with a detail value of \(northward\) is \(north^2\).
Exercise \(\PageIndex{2}\)
Consider several values for n, and show that the number of orbitals for each n is \(n^2\).
Exercise \(\PageIndex{three}\)
Construct a tabular array summarizing the allowed values for the breakthrough numbers n, \(l\), and \(m_l\). for free energy levels i through 7 of hydrogen.
Exercise \(\PageIndex{four}\)
The annotation 3d specifies the quantum numbers for an electron in the hydrogen cantlet. What are the values for n and \(50\) ? What are the values for the energy and angular momentum? What are the possible values for the magnetic quantum number? What are the possible orientations for the athwart momentum vector?
The hydrogen atom wavefunctions, \(\psi (r, \theta , \varphi )\), are called atomic orbitals. An atomic orbital is a office that describes one electron in an cantlet. The wavefunction with n = ane, \(50=ane\), and \(m_l\) = 0 is chosen the 1s orbital, and an electron that is described by this function is said to be "in" the ls orbital, i.e. take a 1s orbital state. The constraints on \(n\), \(50)\), and \(m_l\) that are imposed during the solution of the hydrogen cantlet Schrödinger equation explicate why in that location is a single 1s orbital, why at that place are three 2p orbitals, five 3d orbitals, etc. We will encounter when we consider multi-electron atoms in Chapter 9 that these constraints explicate the features of the Periodic Table. In other words, the Periodic Table is a manifestation of the Schrödinger model and the physical constraints imposed to obtain the solutions to the Schrödinger equation for the hydrogen atom.
Visualizing the variation of an electronic wavefunction with \(r\), \(\theta\), and \(\varphi\) is important because the absolute foursquare of the wavefunction depicts the accuse distribution (electron probability density) in an atom or molecule. The charge distribution is primal to chemistry because it is related to chemic reactivity. For example, an electron scarce role of one molecule is attracted to an electron rich region of another molecule, and such interactions play a major part in chemical interactions ranging from exchange and addition reactions to protein folding and the interaction of substrates with enzymes.
Visualizing wavefunctions and charge distributions is challenging because it requires examining the beliefs of a function of iii variables in iii-dimensional space. This visualization is made easier by considering the radial and athwart parts separately, but plotting the radial and angular parts separately does not reveal the shape of an orbital very well. The shape tin can be revealed better in a probability density plot. To brand such a three-dimensional plot, dissever space up into small volume elements, calculate \(\psi^* \psi \) at the heart of each volume element, and then shade, stipple or color that volume element in proportion to the magnitude of \(\psi^* \psi \). Practice not confuse such plots with polar plots, which look similar.
Probability densities also can exist represented by contour maps, equally shown in Figure \(\PageIndex{one}\).
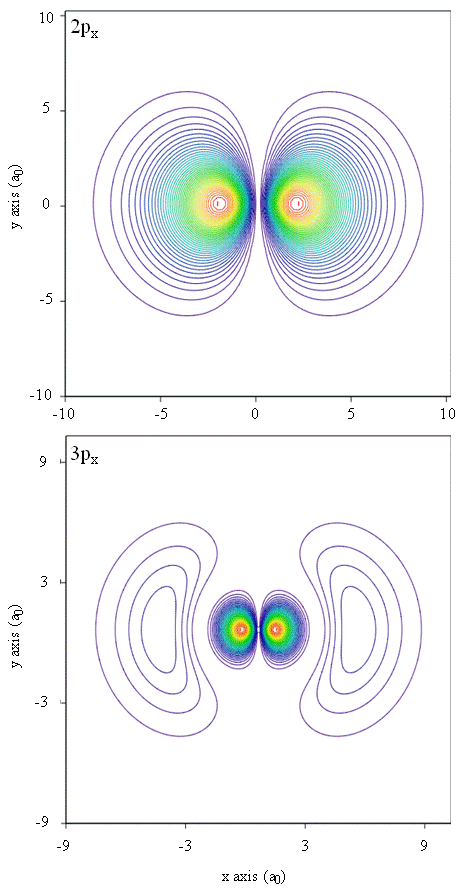
Some other representational technique, virtual reality modeling, holds a keen deal of promise for representation of electron densities. Imagine, for case, existence able to experience electron density as a forcefulness or resistance on a wand that you movement through three-dimensional space. Devices such as these, chosen haptic devices, already exist and are being used to represent scientific data. Similarly, wouldn't it be interesting to "wing" through an diminutive orbital and feel changes in electron density as color changes or cloudiness changes? Specially designed rooms with 3D screens and "smart" glasses that provide feedback about the direction of the viewer's gaze are currently being developed to allow us to experience such sensations.
Methods for separately examining the radial portions of atomic orbitals provide useful information well-nigh the distribution of charge density within the orbitals. Graphs of the radial functions, \(R(r)\), for the 1s, 2s, and 2p orbitals plotted in Figure \(\PageIndex{2}\).
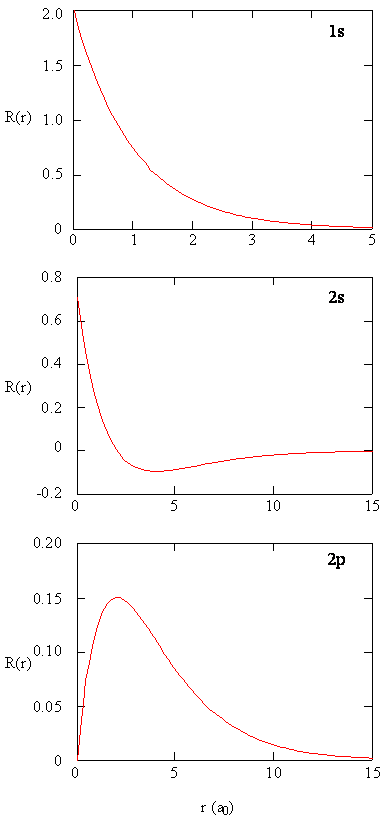
The 1s function in Figure \(\PageIndex{2}\) starts with a high positive value at the nucleus and exponentially decays to essentially zip after v Bohr radii. The high value at the nucleus may be surprising, simply as we shall run across later, the probability of finding an electron at the nucleus is vanishingly minor.
Next notice how the radial function for the 2s orbital, Figure \(\PageIndex{2}\), goes to zip and becomes negative. This behavior reveals the presence of a radial node in the office. A radial node occurs when the radial part equals zero other than at \(r = 0\) or \(r = ∞\). Nodes and limiting behaviors of atomic orbital functions are both useful in identifying which orbital is being described past which wavefunction. For example, all of the due south functions have non-zero wavefunction values at \(r = 0\), but p, d, f and all other functions go to zero at the origin. It is useful to call up that there are \(north-ane-l\) radial nodes in a wavefunction, which means that a 1s orbital has no radial nodes, a 2s has one radial node, then on.
Do \(\PageIndex{v}\)
Examine the mathematical forms of the radial wavefunctions. What feature in the functions causes some of them to go to zero at the origin while the s functions do not go to zero at the origin?
Practise \(\PageIndex{6}\)
What mathematical feature of each of the radial functions controls the number of radial nodes?
Exercise \(\PageIndex{seven}\)
At what value of r does the 2s radial node occur?
Exercise \(\PageIndex{8}\)
Make a table that provides the free energy, number of radial nodes, and the number of angular nodes and total number of nodes for each office with north = 1, 2, and 3. Identify the relationship between the energy and the number of nodes. Identify the relationship between the number of radial nodes and the number of angular nodes.
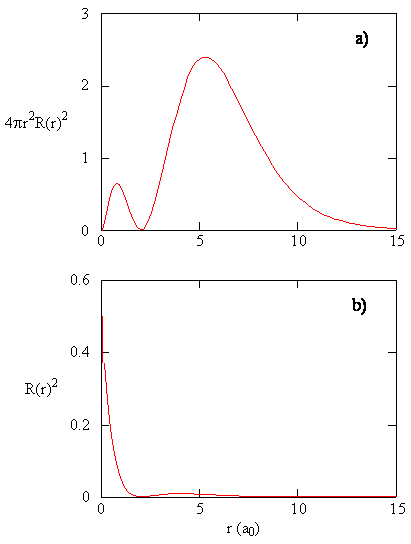
The quantity \(R (r) ^* R(r)\) gives the radial probability density; i.e., the probability density for the electron to be at a indicate located the distance \(r\) from the proton. Radial probability densities for 3 types of atomic orbitals are plotted in Figure (\PageIndex{three}\).
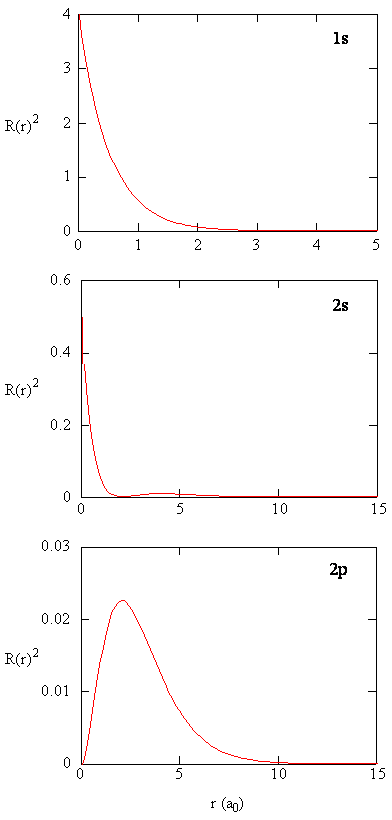
When the radial probability density for every value of r is multiplied past the expanse of the spherical surface represented by that particular value of r, we become the radial distribution function. The radial distribution function gives the probability density for an electron to be found anywhere on the surface of a sphere located a distance r from the proton. Since the area of a spherical surface is \(four \pi r^two\), the radial distribution function is given by \(four \pi r^2 R(r) ^* R(r)\).
Radial distribution functions are shown in Effigy \(\PageIndex{four}\). At small values of r, the radial distribution function is low because the small surface surface area for small radii modulates the high value of the radial probability density function near the nucleus. Every bit we increase \(r\), the surface area associated with a given value of r increases, and the \(r^2\) term causes the radial distribution role to increase even though the radial probability density is get-go to decrease. At large values of \(r\), the exponential decay of the radial function outweighs the increment acquired past the \(r^2\) term and the radial distribution office decreases.
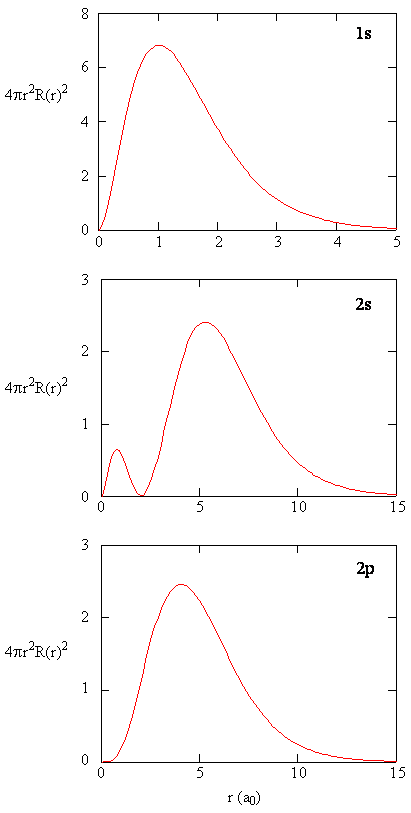
Exercise \(\PageIndex{ix}\)
Write a quality comparison of the radial part and radial distribution role for the 2s orbital. Run into Effigy (\PageIndex{5}\)
Source: https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Book%3A_Quantum_States_of_Atoms_and_Molecules_(Zielinksi_et_al)/08%3A_The_Hydrogen_Atom/8.02%3A_The_Wavefunctions
Posted by: lynnfouty1959.blogspot.com



0 Response to "How To Draw Radial Wave Function"
Post a Comment